Methane decomposition (MD)
reaction, also known as methane
pyrolysis, allows the conversion
of methane from natural gas or
biomethane into solid carbon and
hydrogen with high purity:
CH4 → C (s) + 2H2 ΔH0 = 75.3 kJ mol-1
This reaction has the unique feature
of being 100% selective. Apart from
allowing the swift decarbonization
of the energy, when biomethane is
used, this reaction has the power to
remove CO2 from the atmosphere
as it produces H2 at very competitive
costs. This technology enables
using the present storage and
distribution infrastructure for
natural gas and produces H2 to be
used locally as a fuel for electricity/
heat or as feedstock for chemical
industries (steel production, ammonia
synthesis, reversal petrochemistry,
etc.).
Considering only the price of the raw
materials, H2 from the decomposition
of natural gas costs 1.9 €/kg, while
biomethane-derived H2 costs 2.2
€/kg. At the present prices of CO2
allowances, >60 €/ton, this process
saves 0.54 €/kg of H2. The MD of
biomethane removes CO2 from the
atmosphere. Assuming a cost for the
direct air capture of CO2 of 150 €/ton
and for its sequestration of 50/ton,
per 1 kg of H2 produced, 0.60 € are
saved in capture and sequestration
of CO2.
The industrialization of catalytic MD
has been hindered so far by the
extremely fast catalyst deactivation,
which is caused by the inevitable
coverage of catalytic sites by the
formed solid carbon. Competing
institutions/companies are developing
high temperatures MD processes
involving either metal liquid reactors
or reactors using carbon catalyst
particles; however, these approaches
are energy-intensive, dangerous to
operate and display low catalytic
activity.
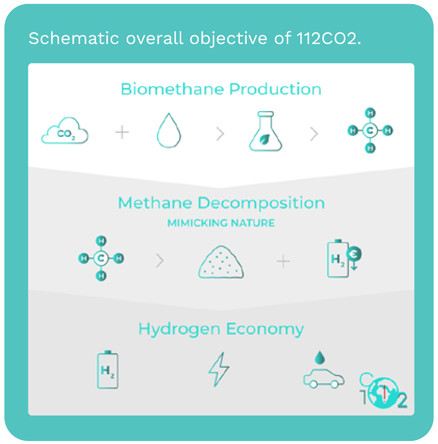 112CO2, a FET-Proactive project,
aims at developing a disruptive low
temperature (ca. 550 °C) methane
decomposition process, using
abundant and cheap metallic
catalysts. Briefly, the designed
reactor uses Ni-based catalysts,
which are very active but need to be
cyclically regenerated. It is expected
to reach >0.45 gH2 gCat
-1 h-1 and
stable for at least 10 000 h.
112CO2, a FET-Proactive project,
aims at developing a disruptive low
temperature (ca. 550 °C) methane
decomposition process, using
abundant and cheap metallic
catalysts. Briefly, the designed
reactor uses Ni-based catalysts,
which are very active but need to be
cyclically regenerated. It is expected
to reach >0.45 gH2 gCat
-1 h-1 and
stable for at least 10 000 h.
The project, which has started in
September 2020, gathers some of
the finest EU research laboratories
and companies, including University
of Porto, author of this new MD
concept; Pixel Voltaic Lda., which
is a spin-off company from UPorto,
responsible for designing the process
lab prototype; CSIC to synthetize
the catalysts; DLR to develop proton
conducting ceramics for efficient and
cost-effective H2 purification; EPFL
responsible for the dissemination
activities; Paul Wurth S.A. and Quantis
for performing life cycle assessment
and economic analysis.
As explained by Adélio Mendes,
professor at the University of Porto
and project coordinator: "preliminary
results allowed to reach a maximum
catalytic activity of 3 gH2 gCat
-1 h-1 and
proved that the cyclic regeneration
allows keeping the catalyst at its
maximum catalytic activity. Initial
experiments demonstrated worldrecord
stabilities, using a compact
reactor loaded with commercial
and non-optimized catalysts."
112CO2 also proposes an ambitious
communication strategy, aiming
to involve stakeholders, investors,
researchers, youngsters, and students
for this emergent technology.
112CO2 project has received funding from the European Union's Horizon 2020 research and innovation Programme under the grant agreement No 952219.